Description
Short Pointer/Headline: Pioneering IV Cannula Technology for Safety, Comfort, and Clinical Confidence.
Manufacturing Details: B. Braun is a global leader in healthcare solutions, renowned for its commitment to safety and innovation. B. Braun’s IV cannulas are engineered with precision in state-of-the-art facilities, adhering to the highest international quality and safety standards. Their products are clinically proven to be safe and effective, providing healthcare professionals with a reliable tool for intravenous access.
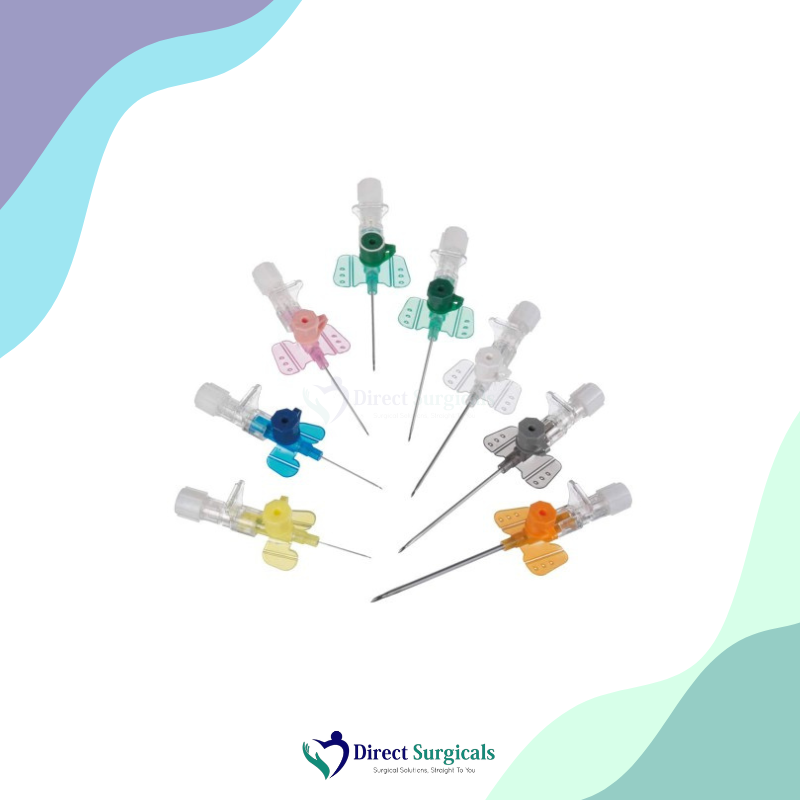
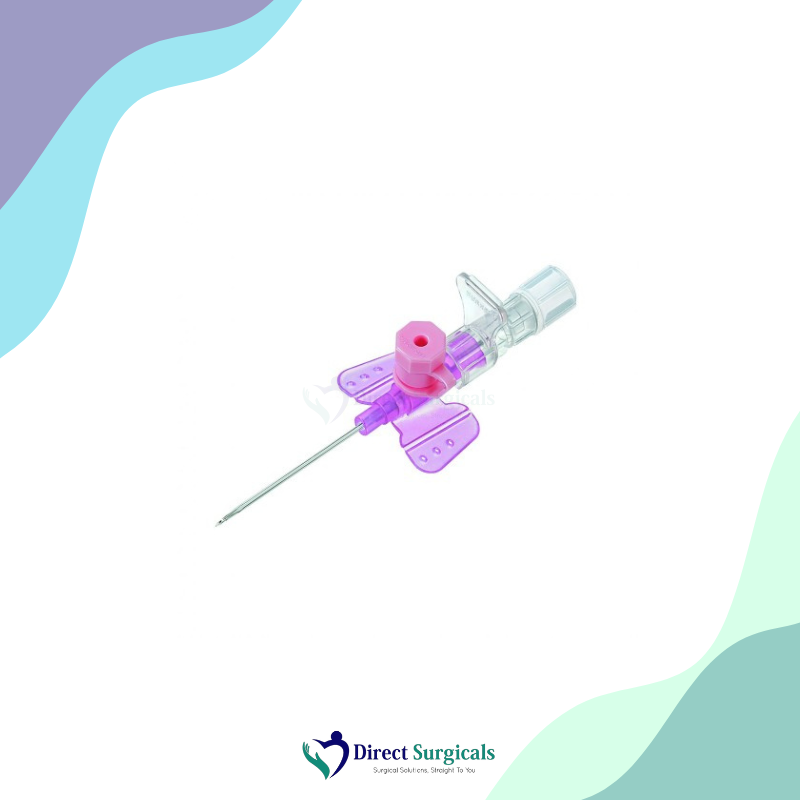
Material Details: The catheter is made from a highly biocompatible material, such as FEP (Fluorinated Ethylene Propylene) or Polyurethane, which is designed to be flexible yet strong. The needle is made from high-quality, surgical-grade stainless steel with a siliconized surface for smooth penetration. The hub and wings are made from medical-grade, non-toxic plastic, with a clear, transparent design to allow for easy visualization of blood flow.
Usability: B. Braun IV cannulas are sterile, single-use devices intended for creating a peripheral intravenous line for infusions, blood transfusions, and drug administration. Their design focuses on making the insertion process as smooth and pain-free as possible for the patient. The color-coded hubs follow the international standard, making it easy for clinicians to quickly identify the gauge size. Many of B. Braun’s cannulas feature a safety mechanism that automatically shields the needle tip upon withdrawal, significantly reducing the risk of needlestick injuries.
- → For single-use only. Do not reuse or resterilize.
- → Do not use if the sterile packaging is damaged or if the protective cap is loose.
- → The cannula should be secured firmly with a dressing or tape after insertion to prevent dislodgement.
- → Aseptic technique must be maintained throughout the insertion process.
- → Dispose of the entire unit in a designated sharps container immediately after use.
- → Safety Mechanism: Many models feature an integrated safety clip or shield that covers the needle tip upon withdrawal, actively preventing needlestick injuries.
- → Thin-Wall Technology: This technology allows for a larger internal diameter, leading to higher flow rates and less trauma to the vein.
- → Triple-Bevel Needle: The ultra-sharp, three-bevel needle tip is designed for smooth, low-friction venipuncture, minimizing patient pain.
- → Universal Color Coding: The hubs are color-coded according to the ISO standard, ensuring quick and easy identification of the gauge size.
- → Wide Range of Sizes: Available in a comprehensive range of gauges (e.g., 14G to 26G) and lengths to suit various patient needs and clinical applications.
- → Wings for Fixation: Some models are equipped with flexible wings that provide secure fixation and reduce the risk of the cannula moving.
- → Product Name: (e.g., Vasofix Safety, Introcan Safety)
- → Catheter Material: FEP or Polyurethane
- → Needle Material: Surgical-grade stainless steel, siliconized
- → Hub Material: Medical-grade plastic, color-coded
- → Sterilization: ETO (Ethylene Oxide)
- → Packaging: Individually blister-packed, sterile.
- → Available Sizes: A wide range of gauges and lengths.
- → ISO 13485: Manufactured under a certified quality management system.
- → CE Certified: Meets the European Union's health, safety, and environmental protection standards.
Target Audience Application: B. Braun IV cannulas are the preferred choice for doctors, nurses, and other healthcare professionals in hospitals, surgical centers, and emergency rooms. They are used for a wide range of procedures requiring intravenous access.
Product Description: Romsons IV Cannulas
Short Pointer/Headline: Reliable IV Cannulas for Safe and Effective Intravenous Access.
Manufacturing Details: Romsons is a well-known and trusted brand in the medical devices and consumables industry, recognized for its commitment to quality. Their IV cannulas are manufactured in a controlled environment to ensure sterility and are packaged with a focus on hygiene and convenience. The production process adheres to essential quality standards, guaranteeing a safe and reliable product.
Material Details: The catheter is made from a high-quality, biocompatible material such as PTFE (Polytetrafluoroethylene), which provides a smooth surface for easy insertion. The needle is made from Japanese steel, known for its sharpness and durability. The hub, wings, and protective cap are made from medical-grade, non-toxic plastic, with the hub being transparent to allow for flashback confirmation.
Usability: Romsons IV cannulas are sterile, single-use devices used for creating a peripheral intravenous line. The user follows standard aseptic technique to insert the cannula into a vein. The sharp, beveled needle and smooth catheter surface ensure a comfortable venipuncture for the patient. The color-coded hubs provide an easy way to identify the gauge size, which is a standard feature across all major brands. The built-in injection port (in some models) allows for easy administration of medication without needing to prick the patient again.
- → For single-use only. Do not reuse.
- → Do not use if the sterile packaging is damaged.
- → The cannula must be secured properly with a dressing after insertion.
- → Ensure the venipuncture site is clean and disinfected before insertion.
- → The entire unit, including the needle and catheter, should be disposed of in a sharps container after use.
- → Sharp Needle: The Japanese steel needle with a sharp, beveled tip ensures a smooth and less painful venipuncture.
- → Biocompatible Catheter: The PTFE catheter material is designed to be flexible and minimizes the risk of irritation or damage to the vein.
- → Color-Coded Hub: The hubs are color-coded according to the ISO standard for quick and easy identification of the gauge size.
- → Injection Port: Many models feature a flash-back chamber and an injection port with a non-return valve, allowing for easy administration of intermittent doses of medication.
- Wings for Secure Fixation: Flexible wings provide a stable platform for securing the cannula to the patient's skin.
- → Product Name: (e.g., R-Cath, Romsons Cannula)
- → Catheter → Material: PTFE
- → Needle Material: Japanese steel
- → Hub Material: Medical-grade plastic, color-coded
- → Sterilization: ETO (Ethylene Oxide)
- → Packaging: Individually blister-packed, sterile.
- → Available Sizes: A wide range of gauges (e.g., 14G to 26G) and lengths
- Manufactured under a certified quality management system, adhering to all relevant medical device standards.
Target Audience Application: Romsons IV cannulas are an essential item for hospitals, clinics, and diagnostic labs. They are widely used by doctors, nurses, and paramedics for all procedures requiring intravenous access.